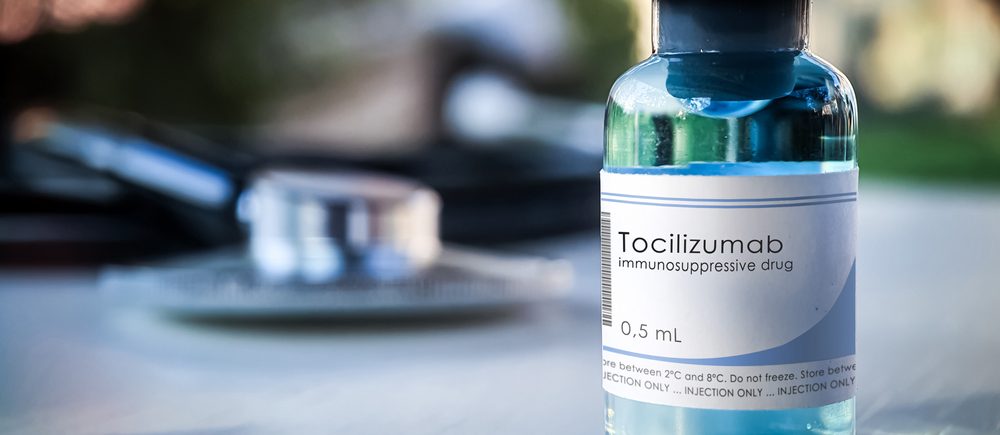
The US Food and Drug Administration said it has authorized emergency use of Actemra to treat adult and pediatric COVID-19 patients in hospitals, and an emergency use authorization has been issued to Genentech, a unit of Roche Holdings Inc.
The Food and Drug Administration said the drug could be used to treat patients receiving corticosteroids who need oxygen support, ventilators or extracorporeal membrane oxidation.
Genentech said in a separate statement that the emergency use authorization is based on the results of four randomized controlled studies that evaluated the efficacy of Actemra for the treatment of Covid-19 in more than 5,500 hospital patients.
The Food and Drug Administration said Actemra is not approved for use in COVID-19 patients outside of hospitals.
The Food and Drug Administration added that clinical trials showed that the drug reduces the risk of death and the time it takes for patients in hospitals to recover.
 Noor Trends News, Technical Analysis, Educational Tools and Recommendations
Noor Trends News, Technical Analysis, Educational Tools and Recommendations